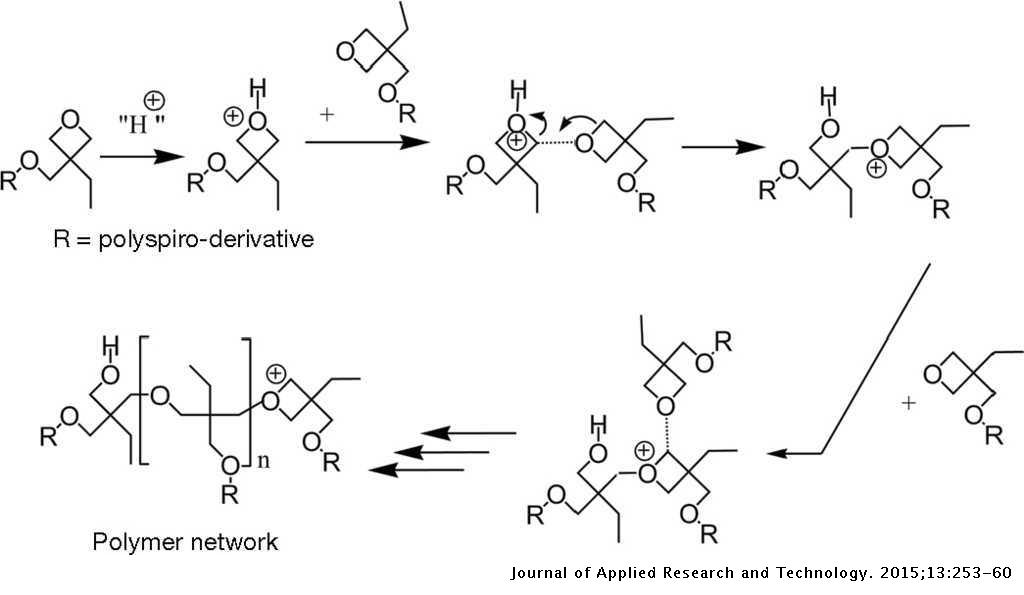
The I-V characteristics of organic hole-only devices based on crosslinked hole-transport layer | Journal of Applied Research and Technology. JART

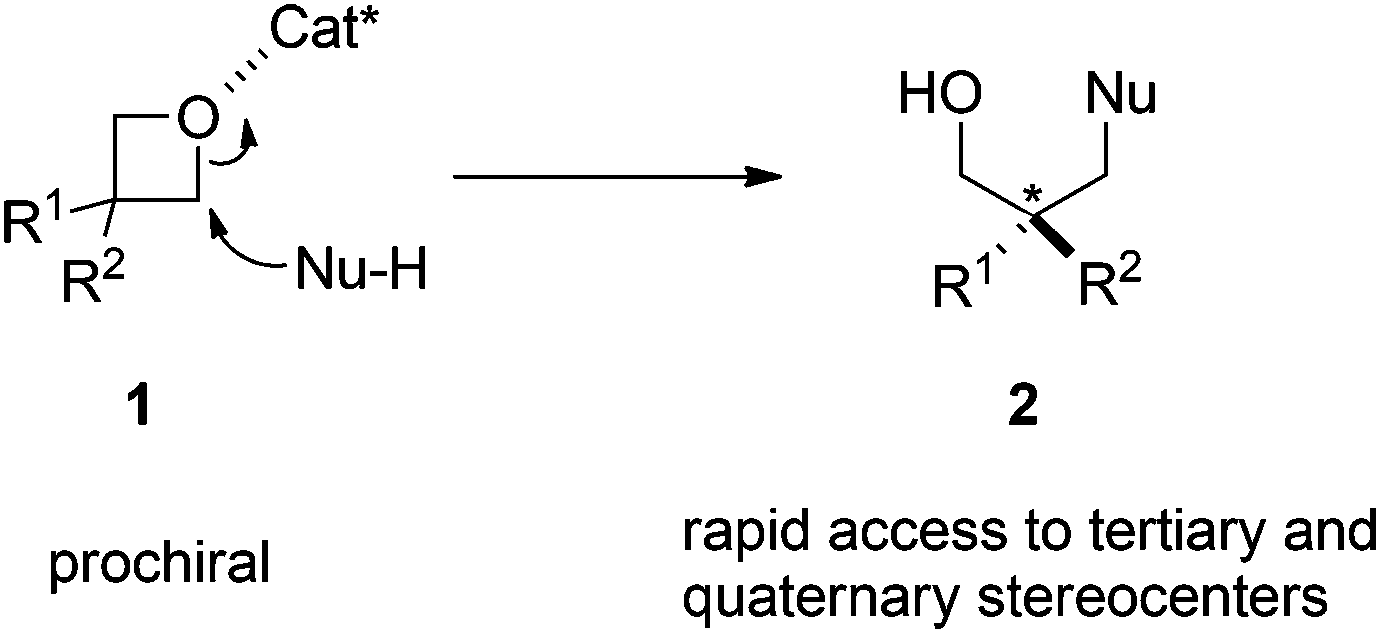
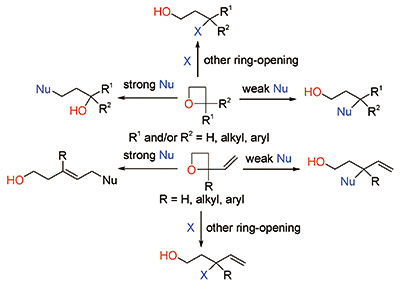
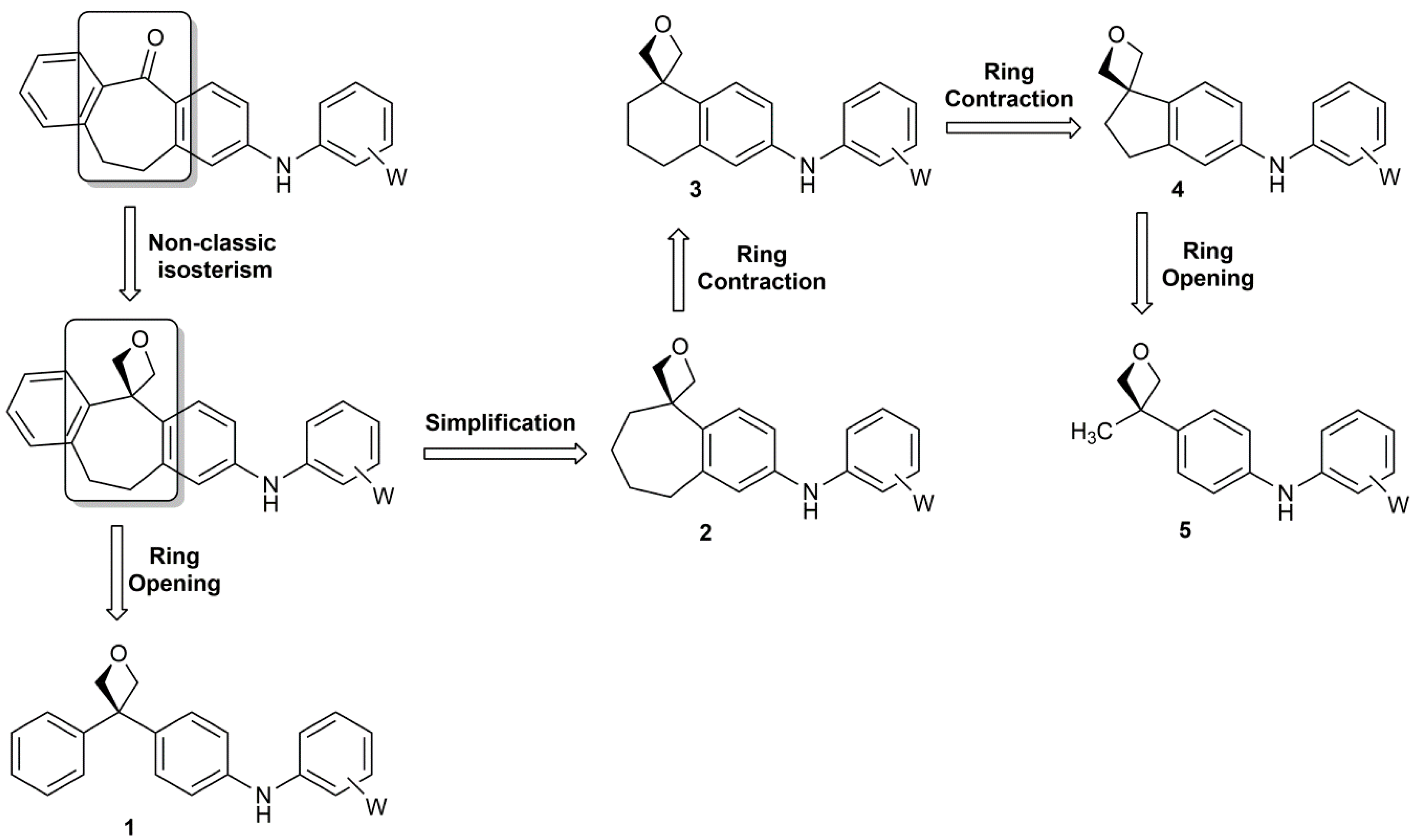
Enantioselective Oxetane Ring Opening with Chloride: Unusual Use of Wet Molecular Sieves for the Controlled Release of HCl - Yang - 2016 - Angewandte Chemie International Edition - Wiley Online Library
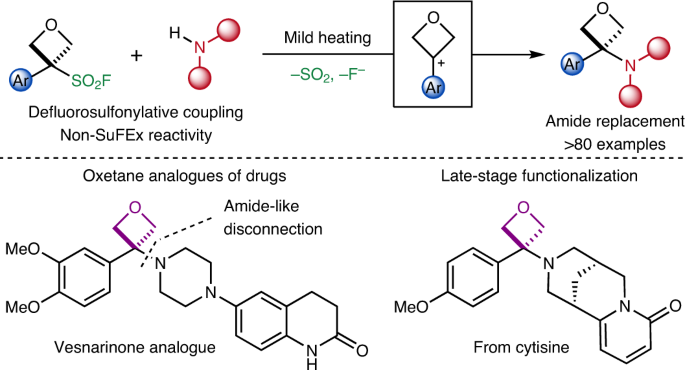
Amino-oxetanes as amide isosteres by an alternative defluorosulfonylative coupling of sulfonyl fluorides | Nature Chemistry

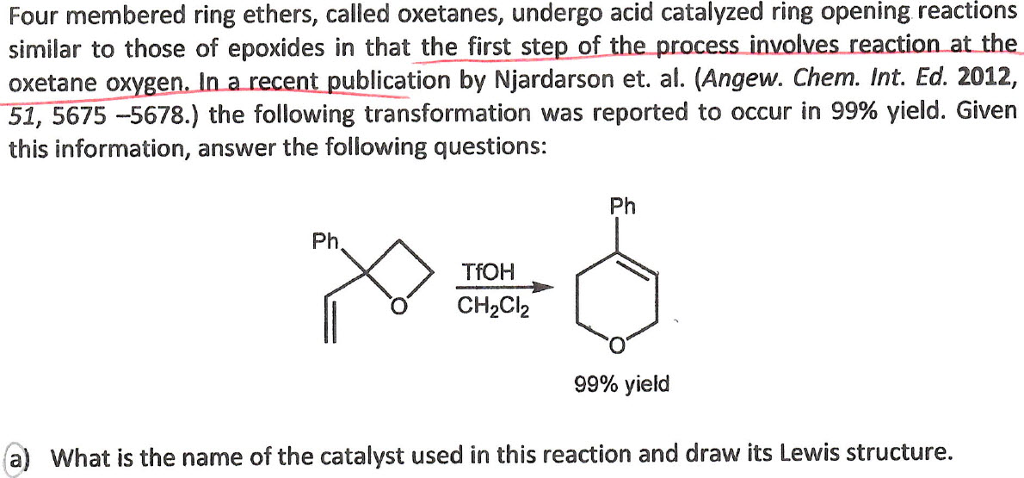
Mild C–C Bond Formation via Lewis Acid Catalyzed Oxetane Ring Opening with Soft Carbon Nucleophiles - Huang - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Cationic ring opening polymerization of oxetane proceeds through the... | Download Scientific Diagram

Polymers | Free Full-Text | α,ω-Epoxide, Oxetane, and Dithiocarbonate Telechelic Copolyolefins: Access by Ring-Opening Metathesis/Cross-Metathesis Polymerization (ROMP/CM) of Cycloolefins in the Presence of Functional Symmetric Chain-Transfer Agents

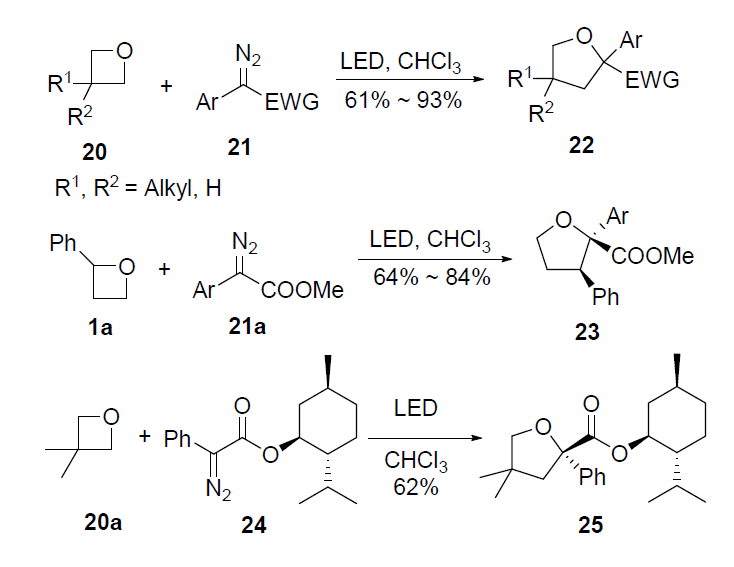
Radical Ring-Opening of Oxetanes Enabled by Co-Catalysis | Organic Chemistry | ChemRxiv | Cambridge Open Engage

Ring opening polymerization of oxetane by the use of a montmorillonite clay as catalyst - ScienceDirect
![PDF] A Direct Synthesis of Highly Substituted π-Rich Aromatic Heterocycles from Oxetanes. | Semantic Scholar PDF] A Direct Synthesis of Highly Substituted π-Rich Aromatic Heterocycles from Oxetanes. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d02caafa010dac63526fcccc13673b75609c7a9f/1-Figure1-1.png)
PDF] A Direct Synthesis of Highly Substituted π-Rich Aromatic Heterocycles from Oxetanes. | Semantic Scholar